
Index
GAO Software Provides Easy Integration with API
Case Studies of RFID, IoT & Drone Applications
GAO RFID Systems & Hardware for Medical Equipment and Supplies Manufacturing Industry
GAO Makes Efforts to Satisfy Customers
GAO Has Served Medical Equipment and Supplies Manufacturing Industry Extensively
Overview
The Medical Equipment and Supplies Manufacturing Industry is a sector dedicated to the production of a wide range of products used in healthcare settings. This industry plays a critical role in supporting medical professionals and improving patient care by manufacturing various devices, instruments, and supplies. Here are some key aspects of this industry, this industry encompasses a broad array of products, including medical devices (e.g., surgical instruments, diagnostic equipment, imaging devices), disposable supplies (e.g., gloves, syringes, bandages), and durable medical equipment (e.g., wheelchairs, hospital beds). The COVID-19 pandemic, for example, led to a surge in demand for items like ventilators, personal protective equipment (PPE), and diagnostic tests. There is a growing emphasis on sustainability within the industry, including the development of eco-friendly products, recycling initiatives, and reducing waste in the manufacturing process.
GAO’s RFID, BLE, IoT, and drone technologies have helped its customers in Medical Equipment and Supplies Manufacturing Industry to improve their work processes, their operations and productivity by better management of their staff, materials and operational equipment such as CNC Machining Centers, Injection Moulding Machines, Laser Cutting and Welding Machines, Extrusion Machines, Cleanrooms, 3D Printers, Assembly Lines, Testing and Inspection Equipment, Sterilization Equipment, Packaging Machinery, Quality Assurance and Testing Equipment, Metal Fabrication Equipment, Ultrasonic Cleaners, Particle Counters, Lab Equipment, Material Handling Equipment, Welding Equipment, CAD/CAM Software, Environmental Monitoring Systems, Pipetting and Dispensing Equipment, Mixing and Blending Equipment, Sewing Machines, Labelling and Barcoding Machines, Material Testing Instruments, Safety and Personal Protective Equipment (PPE).
Ranked as one of the top 10 global RFID suppliers, GAO RFID Inc. is based in New York City, U.S. and Toronto, Canada. GAO offers a comprehensive selection of UHF, HF (including NFC) and LF RFID (radio frequency identification) readers and tags, BLE (Low Energy Bluetooth) gateways and beacons, and various RFID and BLE systems such as people tracking, asset tracking, access control, parking control, fleet management, WIP (work in progress), traceability. Such RFID and BLE products and systems, as well as its IoT and drone technologies, have been successfully deployed for Medical Equipment and Supplies Manufacturing Industry. Its sister company, GAO Tek Inc. https://gaotek.com, is a leading supplier of industrial or commercial testers and analyzers, drones, and network products.
The targeted markets of both GAO RFID Inc. and GAO Tek Inc. are North America, particularly the U.S., Canada, Mexico, and Europe. As a result, this website gaorfid.com is offered in English and other major languages of North America and Europe such as Spanish, French, German, Italian, Polish, Ukrainian, Romanian, Russian, Dutch, Turkish, Greek, Hungarian, Swedish, Czech, Portuguese, Serbian, Bulgarian, Croatian, Danish, Finnish, Norwegian, Slovak, Catalan, Lithuanian, Bosnian, Galician, Slovene, Latvian, Estonian, Welsh, Icelandic, and Irish.
Applications & Benefits of GAO’s RFID, BLE, IoT & Drones for Medical Equipment and Supplies Manufacturing Industry
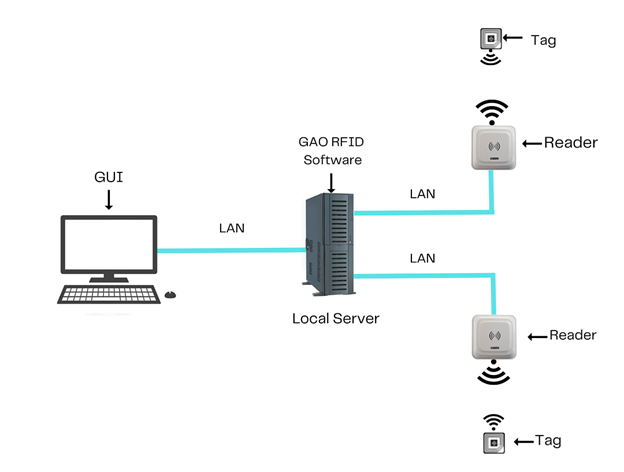
To satisfy its customers, GAO’s RFID or RFID Systems for Medical Equipment and Supplies Manufacturing Industry are offered in 2 versions. One version is that its software is running on a local server that normally is on our client’s premises, and another version runs in the cloud. The cloud server could be GAO’s cloud server, client’s own cloud server or a cloud server from one of the leading cloud server providers such as Amazon Web Services (AWS), Microsoft Azure, Google Cloud, IBM Cloud (formerly SoftLayer), Oracle Cloud, RedHat, Heroku, Digital Ocean, Cloudflare, Linode and Rackspace. The above illustrates GAO system for Medical Equipment and Supplies Manufacturing Industry Software’s running on a local server.
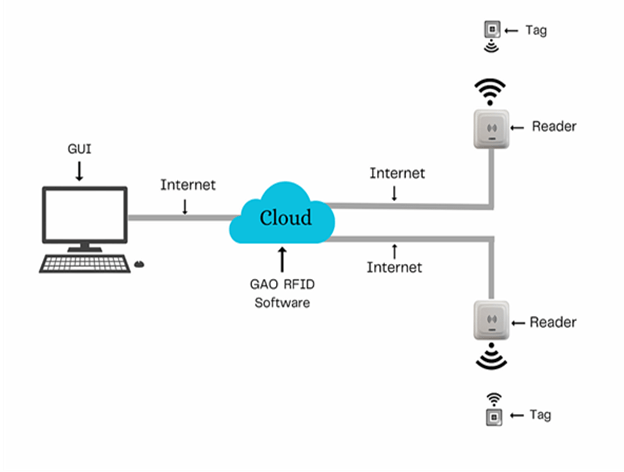
The above illustrates GAO system for Medical Equipment and Supplies Manufacturing Industry with its software running in cloud.
GAO’s RFID and BLE technologies, consisting of RFID readers, RFID tags, BLE gateways, BLE beacons, software, cloud services and their systems, have the following applications in Medical Equipment and Supplies Manufacturing Industry:
- Inventory Management: Our RFID tags can be attached to medical supplies and equipment to monitor stock levels automatically. This helps in reducing stockouts, minimizing overstocking, and ensuring that critical supplies are readily available.
- Asset Tracking: GAO’s RFID is used to track and manage valuable medical equipment, such as surgical instruments, ventilators, and imaging devices. This ensures that equipment is easily located, maintained, and accounted for.
- Quality Control: Our RFID tags can be used to monitor and track the manufacturing process of medical devices and supplies, helping to ensure that quality standards are met at each production stage.
- Patient Identification: GAO’s RFID wristbands or tags are used for patient identification, enhancing safety by ensuring that the right patient receives the right treatment or medication.
- Sterilization Tracking: GAO’s RFID tags can be embedded in sterilization containers to monitor the sterilization process of reusable medical devices and ensure compliance with sterilization protocols.
- Supply Chain Visibility: Our RFID technology enables real-time tracking of medical supplies as they move through the supply chain, from manufacturing facilities to distribution centers and healthcare facilities. This enhances supply chain visibility and reduces the risk of product loss or theft.
- Expiration Date Monitoring: Our RFID can be used to track the expiration dates of medical supplies and alert healthcare providers when items are nearing expiration, helping to reduce waste and ensure the use of safe products.
- Patient Flow Management: GAO’s RFID systems can assist in tracking patient movement within healthcare facilities, streamlining processes such as check-in, waiting room management, and discharge.
- Medical Waste Management: GAO’s RFID tagging can help manage the disposal of hazardous medical waste by tracking the collection and disposal process, ensuring compliance with regulatory guidelines.
- Clinical Trial Management: In clinical research, RFID can be used to track the movement and status of investigational drugs, medical devices, and study participants in clinical trials, improving data accuracy and compliance
GAO’s drone technologies find the following applications in the Medical Equipment and Supplies Manufacturing Industry:
- Inventory Management: Our Drones can be used to perform regular aerial inventory checks, ensuring that the right materials and components are available for production.
- Infrastructure Inspection: GAO’s Drones equipped with cameras and sensors can inspect manufacturing facilities, identifying maintenance needs and ensuring equipment reliability.
- Environmental Monitoring: GAO’s Drones can monitor environmental conditions within manufacturing facilities, helping maintain the controlled environments required for certain processes.
- Asset Protection: GAO’s Drones can be used for security surveillance, protecting valuable equipment and supplies from theft or damage.
- Safety Compliance: They can monitor compliance with safety regulations and ensure that employees follow safety protocols.
- Environmental Compliance: Our Drones can help monitor and report on environmental compliance, ensuring that manufacturing processes meet regulatory requirements.
- Last-Mile Delivery: GAO’s Drones can deliver critical medical supplies and equipment to remote or hard-to-reach areas quickly, including during emergencies.
- Transportation Optimization: GAO’s They can optimize transportation routes for both incoming materials and outgoing finished products, reducing delivery times and costs.
- Product Inspections: GAO’s Drones equipped with high-resolution cameras can perform visual inspections of products during the manufacturing process, identifying defects or deviations from quality standards.
- Data Collection: Our Drones can collect data on manufacturing processes and conditions, contributing to quality assurance efforts.
- Prototype Testing: Our Drones can transport prototypes or experimental products to field testing locations, collecting real-world data for research and development purposes.
- Disaster Relief: In case of natural disasters or emergencies, drones can be used to deliver emergency medical supplies, including critical equipment, medications, and supplies, to affected areas.
- Waste Monitoring: GAO’s Drones can assist in monitoring and managing the disposal of hazardous or medical waste, ensuring compliance with regulations.
- Data Gathering: GAO’s Drones equipped with various sensors can collect data on factors like temperature, humidity, and air quality, which is essential in medical equipment manufacturing to maintain product quality.
- Field Testing: Our Drones can transport prototypes and products to remote locations for testing under real-world conditions, aiding in product development and improvement.
GAO’s IoT technologies, consisting of IoT sensors, sensors networks and systems, find the following applications in the Medical Equipment and Supplies Manufacturing Industry:
- Real-Time Equipment Monitoring: Our IoT sensors on manufacturing equipment monitor their performance, providing real-time data on machine health, usage patterns, and maintenance needs. This ensures optimal equipment uptime and minimizes downtime.
- Supply Chain Optimization: GAO’s IoT-enabled tracking and monitoring of raw materials and components provide insights into inventory levels, location, and condition, helping streamline supply chain management and reduce costs.
- Predictive Maintenance: GAO’s IoT sensors collect data on equipment usage and conditions, allowing predictive maintenance to prevent breakdowns and reduce unplanned downtime.
- Quality Control and Assurance: Our IoT devices and sensors monitor production processes, capturing data on variables like temperature, humidity, and pressure, ensuring product quality and compliance with standards.
- Energy Efficiency: GAO’s IoT systems can optimize energy consumption in manufacturing facilities, reducing operational costs and environmental impact.
- Worker Safety and Health: GAO’s IoT wearables and sensors can monitor workers’ health and safety, ensuring compliance with safety regulations and providing early alerts in case of accidents or health issues.
- Environmental Monitoring: Our IoT devices can continuously monitor environmental conditions within manufacturing facilities, ensuring compliance with stringent requirements for cleanliness and sterility.
- Track and Trace: Our IoT-enabled tracking solutions can monitor the movement of products throughout the manufacturing process and supply chain, enhancing traceability and security.
- Regulatory Compliance: GAO’s IoT systems can help monitor and ensure compliance with regulatory standards, reducing the risk of costly fines and product recalls.
- Data Analytics and Optimization: Our IoT-generated data can be analyzed to identify patterns, trends, and areas for process optimization, leading to more efficient manufacturing operations.
- Patient Records and Medication Management: GAO’s IoT-enabled medical equipment and devices can automatically update patient records, ensuring accurate and real-time documentation of care.
GAO Helps Customers Comply with Standards, Mandates & Regulations of Medical Equipment and Supplies Manufacturing Industry
GAO RFID Inc. has helped many companies in Medical Equipment and Supplies Manufacturing Industry to deploy RFID, BLE, IoT and drone systems and to ensure such deployments complying with the applicable industry standards, mandates and government regulations:
RFID, BLE, IoT, & Drone Standards & Mandates
- EPC Global Gen2
- ISO 18000 Series
- ISO 13485
- FDA UDI (Unique Device Identification)
- Bluetooth 4.0
- IEEE 802.15.1
- ISO/IEEE 11073
- Continua Health Alliance
- MQTT (Message Queuing Telemetry Transport)
- CoAP (Constrained Application Protocol)
- OPC UA (Unified Architecture)
- IEEE 802.11
- Thread
- ASTM International F38
- ISO 21384
- FAA (Federal Aviation Administration) Regulations
US.Government Regulations
- FDA Quality System Regulation (QSR)
- FDA Unique Device Identification (UDI) System
- FDA Medical Device Reporting (MDR) Regulation
- FDA 510(k) Premarket Notification
- FDA Current Good Manufacturing Practice (CGMP) Regulations
- FDA Medical Device Classification and Reclassification
- FDA Investigational Device Exemption (IDE) Regulations
- FDA Premarket Approval (PMA) Regulations
- FDA Post-Market Surveillance and Reporting
- EPA Hazardous Waste Regulations
- OSHA Bloodborne Pathogens Standard
- OSHA Hazard Communication Standard (HazCom)
- DEA Controlled Substances Regulations
- USDA Regulations for Biologics and Blood Products
- FTC Truth in Advertising Regulations
- HIPAA Privacy and Security Rules
Canadian Government Regulations
- Health Canada Medical Devices Regulations
- Health Canada Medical Device Licensing
- Health Canada Good Manufacturing Practices (GMP) Guidelines
- Health Canada Medical Device Establishment Licensing
- Health Canada Medical Device Single Audit Program (MDSAP)
- Health Canada Investigational Testing of Medical Devices
- Health Canada Mandatory Medical Device Incident Reporting (MDPR)
- Health Canada Drug Establishment Licensing for Combination Products
- Health Canada Natural Health Products Regulations (if applicable)
- Health Canada Pest Control Products Regulations (if applicable)
- Health Canada Radiation Emitting Devices Regulations (if applicable)
- Health Canada Controlled Drugs and Substances Regulations (if applicable)
- Transport Canada Regulations for the Transportation of Medical Supplies
- Canadian Intellectual Property Office (CIPO) Patent Regulations (related to intellectual property protection)
- Competition Bureau Regulations
GAO Software Provides Easy Integration with API
GAO’s RFID and BLE software offers a free trial for both the server-based and cloud versions, and offers an API to the important systems in Medical Equipment and Supplies Manufacturing Industry such as:
Personnel Management:
- Software for scheduling and managing shifts, ensuring proper staffing levels.
- Systems to handle employee records, payroll, benefits, and compliance.
- Tools for tracking employee training and certifications, ensuring regulatory compliance.
- Software to monitor and evaluate employee performance and set goals.
- for managing workplace health and safety, including incident reporting.
Equipment Management:
- RFID and IoT solutions to track and manage medical equipment and devices.
- Software to schedule and track maintenance tasks for manufacturing equipment.
- Systems to ensure that measuring and testing equipment is calibrated and accurate.
- Solutions for monitoring and optimizing energy usage in manufacturing facilities.
- Tools for managing the entire lifecycle of equipment, from acquisition to disposal.
Access Control:
- Systems to restrict access to sensitive areas within manufacturing facilities.
- Software to manage who has access to critical data and systems.
- Tools for registering and monitoring visitors to manufacturing facilities.
Warehouse Management:
- Systems for tracking and managing raw materials, work-in-progress, and finished goods.
- Software for efficient order picking, packing, and shipping of medical supplies.
- Solutions for predicting demand and optimizing inventory levels.
- Technology for quickly and accurately scanning and managing inventory.
Supply Chain Management:
- Systems to manage and evaluate supplier relationships.
- Tools for forecasting demand and aligning production and distribution accordingly.
- Solutions for optimizing the transportation and distribution of medical supplies.
- Systems for monitoring and maintaining the temperature of sensitive medical products during transport.
Other Applications:
- Software and tools for ensuring product quality and regulatory compliance.
- Systems to monitor and maintain controlled environments in cleanrooms.
- Health Record (EHR) systems for managing patient data related to medical devices.
- Solutions for tracking and reporting compliance with industry regulations.
- Managing relationships with healthcare customers and distributors.
- Accounting and financial software for managing budgets, costs, and financial reporting.
- Software for product design, prototyping, and testing
GAO has enabled its customers to make use of some of the leading software and cloud services in Medical Equipment and Supplies Manufacturing Industry. Below are some of popular software and cloud services in Medical Equipment and Supplies Manufacturing Industry:
SAP SuccessFactors, Workday HCM, Oracle HCM Cloud, ADP Workforce Now, Infor EAM, IBM Maximo, and Maintenance Connection, Cloud HCM, ADP Workforce Now, IBM Maximo Asset Management in the cloud, Fiix Cloud, and Asset Panda Cloud, HID Global, LenelS2, Manhattan Associates, Kinaxis Rapid Response, and Oracle Supply Chain Management Cloud, Brivo, Kisi, Openpath, and Cloudastructure, and warehouse management cloud services include Blue Yonder, HighJump, and SAP Extended Warehouse Management. Supply chain management is supported by Oracle Cloud SCM and SAP Integrated Business Planning on SAP Cloud Platform, Siemens Teamcenter, Dassault Systèmes SOLIDWORKS, PTC Windchill, Autodesk Fusion 360, and Siemens NX, while cloud services encompass Siemens Teamcenter Cloud, Dassault Systèmes 3DEXPERIENCE on Cloud, PTC Onshape, Autodesk Fusion 360 Cloud, and AWS.
GAO has worked with some of the leading technology companies in Medical Equipment and Supplies Manufacturing Industry in to provide integrated RFID, BLE, IoT and drone solutions to customers. Here are some of the technology leaders in Medical Equipment and Supplies Manufacturing Industry:
IBM, Oracle, Microsoft, SAP, Siemens Healthineers, Cerner, GE Healthcare, Philips Healthcare, McKesson, Epic Systems, Intel, Texas Instruments, Analog Devices, STMicroelectronics, NXP Semiconductors, Renesas Electronics, Maxim Integrated, Microchip Technology, ON Semiconductor, Broadcom, Siemens, Rockwell Automation, Schneider Electric, ABB, Emerson Electric, Honeywell, Yokogawa Electric Corporation, Johnson Controls, Beckhoff Automation, Omron Corporation
Case Studies of RFID, IoT & Drone Applications
Case Studies of RFID Applications
Below are some RFID application cases in Medical Equipment and Supplies Manufacturing industry.
- Boston Scientific uses RFID to track the location of surgical instruments in the operating room. This helps to prevent errors and improve patient safety.
- Medtronic uses RFID to track the inventory of medical devices in their warehouses. This helps them to improve efficiency and reduce costs.
- Johnson & Johnson uses RFID to track the distribution of medical supplies to hospitals and clinics. This helps them to ensure that the right supplies are delivered to the right place at the right time.
- GE Healthcare uses RFID to track the movement of patients through the hospital. This helps them to improve efficiency and reduce wait times.
- Siemens Healthineers uses RFID to track the temperature of medical devices during transport. This helps to prevent damage to the devices and ensure that they are safe to use.
- Welch Allyn uses RFID to track the location of medical equipment in their warehouses. This helps them to improve efficiency and reduce costs.
- 3M uses RFID to track the inventory of medical supplies in their warehouses. This helps them to improve efficiency and reduce costs.
- Maquet uses RFID to track the movement of patients through the operating room. This helps them to improve efficiency and reduce wait times.
- Braun Medical uses RFID to track the temperature of medical devices during transport. This helps to prevent damage to the devices and ensure that they are safe to use.
- Hospira uses RFID to track the location of medical equipment in hospitals and clinics. This helps them to improve efficiency and reduce costs.
- Philips uses RFID to track the inventory of medical devices in their warehouses. This helps them to improve efficiency and reduce costs.
- Baxter uses RFID to track the temperature of medical devices during transport. This helps to prevent damage to the devices and ensure that they are safe to use.
- Philips uses RFID to track the inventory of medical devices in their warehouses. This helps them to improve efficiency and reduce costs.
- Many applications of RFID by GAO can be found here
Case Studies of IoT Applications
Below are some IoT application cases in Medical Equipment and Supplies Manufacturing industry.
- Medtronic uses IoT to monitor the performance of its insulin pumps. This helps them to identify potential problems early on and prevent device failures.
- Johnson & Johnson uses IoT to track the location of its medical devices in the field. This helps them to ensure that the devices are being used properly and that they are not being tampered with.
- GE Healthcare uses IoT to collect data from its medical imaging equipment. This data is used to improve the performance of the equipment and to develop new diagnostic tools.
- Philips uses IoT to monitor the condition of its medical devices in hospitals and clinics. This helps them to prevent equipment failures and ensure patient safety.
- Siemens Healthineers uses IoT to connect its medical devices to a cloud-based platform. This allows doctors and nurses to remotely monitor patients and adjust treatment plans as needed.
- Welch Allyn uses IoT to track the location of its medical equipment in hospitals and clinics. This helps them to improve efficiency and reduce costs.
- 3M uses IoT to monitor the temperature of its medical devices during transport. This helps to prevent damage to the devices and ensure that they are safe to use.
- Maquet uses IoT to track the usage of its medical devices in hospitals and clinics. This helps them to optimize the use of the devices and reduce costs.
- Braun Medical uses IoT to collect data from its medical devices. This data is used to improve the performance of the devices and to develop new features.
- Hospira uses IoT to track the location of its medical supplies in hospitals and clinics. This helps them to ensure that the supplies are always available when needed.
- Siemens Healthineers uses IoT to monitor the condition of its medical devices in hospitals and clinics. This helps them to prevent equipment failures and ensure patient safety.
GAO RFID Systems & Hardware for Medical Equipment and Supplies Manufacturing Industry
GAO RFID Inc. offers the largest selection of BLE gateways, BLE beacons, RFID readers, tags, antenna, printers, and integrated RFID systems for various industries, including Medical Equipment and Supplies Manufacturing Industry.
BLE (Bluetooth Low Energy)
GAO offers advanced BLE gateways:
as well as versatile beacons with such important functions as temperature, humility, vibration and panic button:
GAO’s BLE technology is suitable for many industries, including Medical Equipment and Supplies Manufacturing Industry.
UHF (Ultra High Frequency) RFID
GAO offers the largest selection of UHF RFID readers for various industries, including Medical Equipment and Supplies Manufacturing Industry:
GAO RFID offers the widest choice of UHF RFID tags, labels, badges, wristbands for various industries, including Medical Equipment and Supplies Manufacturing Industry:
and an array of antennas to address different applications:
HF (High Frequency), NFC (Near Field Communications) and LF (Low Frequency) RFID
GAO offers the largest selection of HF, NFC, and LF RFID readers for various industries, including Medical Equipment and Supplies Manufacturing Industry:
HF, NFC and LF RFID tags, labels, badges, wristbands for various industries, including Medical Equipment and Supplies Manufacturing Industry:
and antennas:
GAO also offers RFID printers:
Digital I/O adapters:
and relay controllers:
For embedded applications, GAO offers UHF, HF and LF RFID reader modules:
- Find Your 860-960 MHz RFID Modules
- Find Your 13-56 MHz High Frequency RFID Modules
- Find Your 125 KHz Low Frequency RFID Modules
In collaboration with its sister company GAO Tek Inc, a wide selection of high-quality drones is offered:
The RFID systems by GAO are highly popular for clients in Medical Equipment and Supplies Manufacturing Industry:
Physical asset or operational equipment tracking system:
Assets that can be effectively tracked using GAO’s technologies include
CNC Machining Centers, Injection Molding Machines, Laser Cutting and Welding Machines, Extrusion Machines, Cleanrooms, 3D Printers, Assembly Lines, Testing and Inspection Equipment, Sterilization Equipment, Packaging Machinery, Quality Assurance and Testing Equipment, Metal Fabrication Equipment, Ultrasonic Cleaners, Particle Counters, Lab Equipment, Material Handling Equipment, Welding Equipment, CAD/CAM Software, Environmental Monitoring Systems, Pipetting and Dispensing Equipment, Mixing and Blending Equipment, Sewing Machines, Labeling and Barcoding Machines, Material Testing Instruments, Safety and Personal Protective Equipment (PPE).
People or workers tracking system:
Personnel or people access control system:
Parking or vehicle control system:
Furthermore, GAO provides the customization of RFID tags, RFID readers, BLE beacons and BLE gateways, IoT, drones, and systems and consulting services for Medical Equipment and Supplies Manufacturing Industry and for various industries in all metropolitans in North America, particularly the U.S., Canada and Mexico, and Europe: https://gaorfid.com/services.
GAO Makes Efforts to Satisfy Customers
Large Choice of Products
In order to satisfy the diversified needs of their corporate customers, GAO RFID Inc. and its sister company GAO Tek Inc. together offer a wide choice of RFID, BLE, IoT, drones, testing and measurement devices, and network products.
Overnight Delivery
In order to shorten the delivery to our customers, GAO has maintained a large stock of its products and is able to ship overnight within continental U.S. and Canada, and fast delivery to anywhere in Mexico and Europe from the nearest warehouse.
Local to Our Customers
We are located in both the U.S. and Canada. We travel to customers’ premises if necessary. Hence, we provide a very strong local support to our customers in North America, particularly the U.S., Canada and Mexico, and Europe. Furthermore, we have built partnerships with some integrators, consulting firms and other service providers in different cities to further strengthen our services. Here are some of the service providers in Medical Equipment and Supplies Manufacturing Industry we have worked with to serve our joint customers:
- Cerner
- GE Healthcare
- Siemens Healthineers
- McKesson
- Accenture
- CGI
- Entegris
- Intellectual Capital
- Westwind Technologies
- Everis
- IBM
- Philips
- Agfa HealthCare
- Capgemini
- Cerner
- GE Healthcare
- Siemens Healthineers
- McKesson
- Accenture
- CGI
- Entegris
- Intellectual Capital
- Westwind Technologies
- Everis
- IBM
- Philips
- Agfa HealthCare
- Capgemini
GAO Has Served Medical Equipment and Supplies Manufacturing Industry Extensively
GAO RFID Inc. and its sister company GAO Tek Inc. together offer a wide choice of RFID, BLE, IoT, drone, testing and measurement devices, and network products.
GAO’s products and technologies have helped its customers in Medical Equipment and Supplies Manufacturing Industry to achieve success in
Telemedicine, Additive Manufacturing (3D Printing), IoT and Connectivity, Smart Wearables, Personalized Medicine, Sustainability, Artificial Intelligence (AI), Robotic Surgery, Patient-Centric Design, Blockchain, Precision Medicine, Telehealth, Blockchain in Healthcare, IoMT (Internet of Medical Things), Digital Twin, Remote Patient Monitoring, Nanotechnology, FDA 510(k) Clearance, Healthcare Interoperability, Regulatory Compliance, Human-Centered Design, MHealth (Mobile Health), Regenerative Medicine, HealthTech, EHR (Electronic Health Record) Integration, HIPAA Compliance, Regulatory Affairs, AI-Driven Diagnostics, Patient Data Privacy, Point-of-Care Testing (POCT)
GAO RFID Inc. has deployed RFID, BLE and IoT projects for many companies in Medical Equipment and Supplies Manufacturing Industry, including many in its various divisions such as
- Medical Device Manufacturing: This division encompasses the production of a wide range of medical devices, including diagnostic equipment, surgical instruments, implants (e.g., pacemakers, artificial joints), and patient monitoring devices.
- Medical Equipment Manufacturing: Companies in this sub-industry produce non-electronic medical equipment such as hospital beds, diagnostic imaging equipment (e.g., X-ray machines), and dental equipment.
- Medical Supplies Manufacturing: This includes the manufacturing of disposable medical supplies and consumables like syringes, bandages, gloves, catheters, and wound care products.
- Biotechnology: Biotech companies focus on developing and manufacturing biopharmaceuticals, genetic therapies, and biologically derived medical products, including vaccines and monoclonal antibodies.
- Dental Equipment and Supplies Manufacturing: Specialized sub-industry involved in producing dental equipment, instruments, and supplies used by dentists and oral healthcare professionals.
- Orthopedic and Prosthetic Manufacturing: Companies in this division specialize in orthopedic implants, joint replacements, and prosthetic limbs and devices.
- Pharmaceutical Manufacturing: While pharmaceuticals primarily involve drug production, some medical equipment, like drug delivery devices (e.g., inhalers, insulin pens), are closely related to this industry.
- Optical and Ophthalmic Equipment Manufacturing: This sub-industry produces optical and ophthalmic equipment, including eyeglasses, contact lenses, and ophthalmic surgical instruments.
- Diagnostic Imaging Equipment Manufacturing: Specializes in the manufacturing of diagnostic imaging devices like MRI machines, CT scanners, ultrasound equipment, and radiography systems.
- Laboratory Equipment Manufacturing: Companies within this division produce laboratory instruments, equipment, and consumables used in medical and scientific research.
- Rehabilitation and Assistive Devices Manufacturing: Focuses on the production of devices that aid in rehabilitation, including mobility aids, hearing aids, and assistive technology for individuals with disabilities.
- Surgical and Medical Instrument Manufacturing: Specializes in the production of surgical instruments, endoscopes, and minimally invasive surgical tools used in medical procedures.
- In-Vitro Diagnostics (IVD) Manufacturing: This sub-industry manufactures IVD devices used for analyzing samples such as blood, urine, and tissue to diagnose diseases and conditions.
- Medical Furniture Manufacturing: Produces hospital and healthcare facility furniture, including examination tables, medical carts, and patient seating.
- Emergency Medical Equipment Manufacturing: Companies in this division produce emergency medical equipment such as defibrillators, first aid kits, and trauma care supplies.
- Laboratory Consumables Manufacturing: Specializes in producing consumable items used in laboratory settings, including test tubes, pipettes, and culture dishes.
GAO’s technologies enable its customers in “Medical Equipment and Supplies Manufacturing Industry” to effectively track their workforces such as Medical Device, ISO Standards, Cleanroom, GMP (Good Manufacturing Practices), FDA (U.S. Food and Drug Administration), CE Marking, Validation, Quality Control (QC), Regulatory Compliance, SOP (Standard Operating Procedure), Traceability, Supplier Quality Management, Risk Management, MSDS (Material Safety Data Sheet), Batch Manufacturing Record (BMR), Device Master Record (DMR), CAPA (Corrective and Preventive Actions), Validation Protocol, Bill of Materials (BOM), FDA 510(k) Clearance, Design Control, Shelf Life, Biocompatibility, Usability Testing, and Quality Management System (QMS) Respectively track operational assets such as CNC Machines, Injection Molding Machines, Laser Cutting Machines, Extrusion Machines, Autoclaves, Cleanrooms, 3D Printers, Assembly Lines, Testing and Inspection Equipment, Ultrasonic Cleaners, Sterilizers, Packaging Machinery, Quality Control Tools, Material Handling Equipment, Welding Machines, CAD/CAM Software, Environmental Monitoring Systems, Mixing and Blending Equipment, Barcode Scanners, Lab Equipment, Material Testing Instruments, Pipetting and Dispensing Equipment, Labelling and Barcoding Equipment, Robotics and Automation, and Particle Counters.
Here are some of the leading companies in Medical Equipment and Supplies Manufacturing Industry GAO has served:
- Boston Scientific
- Medtronic
- Johnson & Johnson
- GE Healthcare
- Siemens Healthineers
- Abbott Laboratories
- Hill-Rom
- BD
- Stryker
- Medline Industries
- Philips
- Intuitive Surgical
- Zimmer Biomet
- Abiomed
- Edwards Lifesciences
- Cardinal Health
- Smith & Nephew
- Teleflex
- Cook Medical
- Baxter
- Welch Allyn
- 3M
- Maquet
- Braun Medical
- Hospira
- Siemens Healthineers
- Johnson & Johnson
- Philips
- Baxter
- Medtronic
- Siemens Healthineers
- Philips
- Medtronic
- Johnson & Johnson
- GE Healthcare





























You Are Invited to Contact Us!
If you are interested in our products, services or partnering with us, please feel free to contact us by filling out this form:
or email us at sales@gaorfid.com
