Index
GAO’s assist clients with pharmaceutical and drug manufacturing standards and regulations
GAO software provides easy API integration
Case Studies of RFID Applications
GAO RFID Systems & Hardware for the manufacture of pharmaceuticals and medicines
GAO has served the pharmaceutical and medicine manufacturing extensively
Related Products & Systems on Other Pages on This Website
Employee & Attendance Access Control System
Return & Pallet Asset Tracking – GAO RFID
Work In Process WIP Asset Tracking System
People Tracking System for Manufacturing Facilities
GAO RFID Medical Diagnostic Laboratories Asset Management System – GAO RFID
BLE | Bluetooth Low Energy | BLE Gateways & Beacons – GAO RFID
RFID Readers | Buy RFID Readers | RFID Reader Writers – GAO RFID
RFID Tags | Buy RFID Tags – GAO RFID
Overview
The pharmaceutical and medicine manufacturing industry is a sector dedicated to the production, development and commercialization of medicines and health products. This industry is responsible for creating treatments and solutions for diseases and medical disorders, and ranges from the research and development of new drugs to the manufacture and distribution of pharmaceuticals worldwide. Pharmaceuticals and medicines are essential for human health and well-being, which is why this industry plays a fundamental role in society.
GAO’s RFID, BLE, IoT and drone technologies have helped its clients in the manufacture of pharmaceutical products and medicines to improve their work processes, their operations and productivity through better management of their personnel, materials and operational equipment, such as: Reactors and mixing tanks, centrifuges, dryers, mills and mixers, purification systems, packaging and labeling systems, sterilization equipment, quality control equipment, automation and control equipment, refrigeration systems.
Ranked as one of the top 10 global RFID providers, GAO RFID Inc. is headquartered in New York City, USA and Toronto, Canada. GAO offers a wide selection of UHF, HF (including NFC), and LF RFID (Radio Frequency Identification) readers and tags, BLE (Bluetooth Low Energy) gateways and beacons, and various RFID and BLE systems such as people tracking, asset tracking, access control, parking control, fleet management, WIP (work in progress), traceability. Said RFID and BLE products and systems, as well as their IoT and drone technologies, have been successfully implemented for the manufacture of pharmaceuticals and medicines.
Applications and benefits of GAO’s RFID, BLE, IoT and drones for the manufacture of pharmaceuticals and medicines
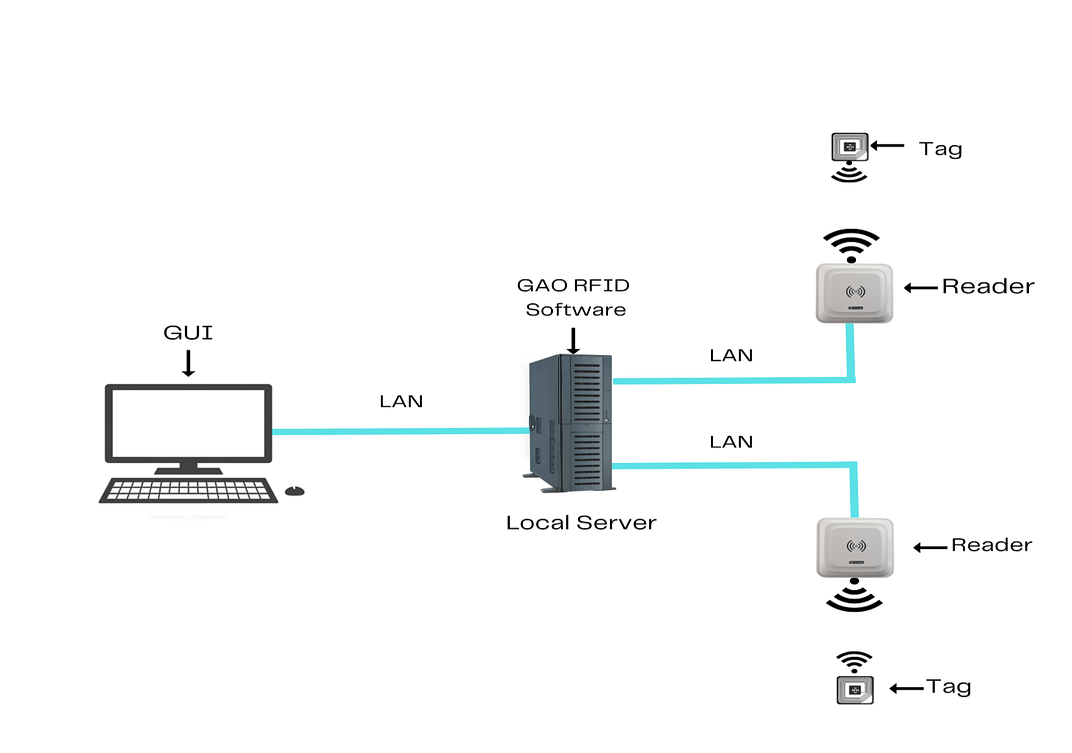
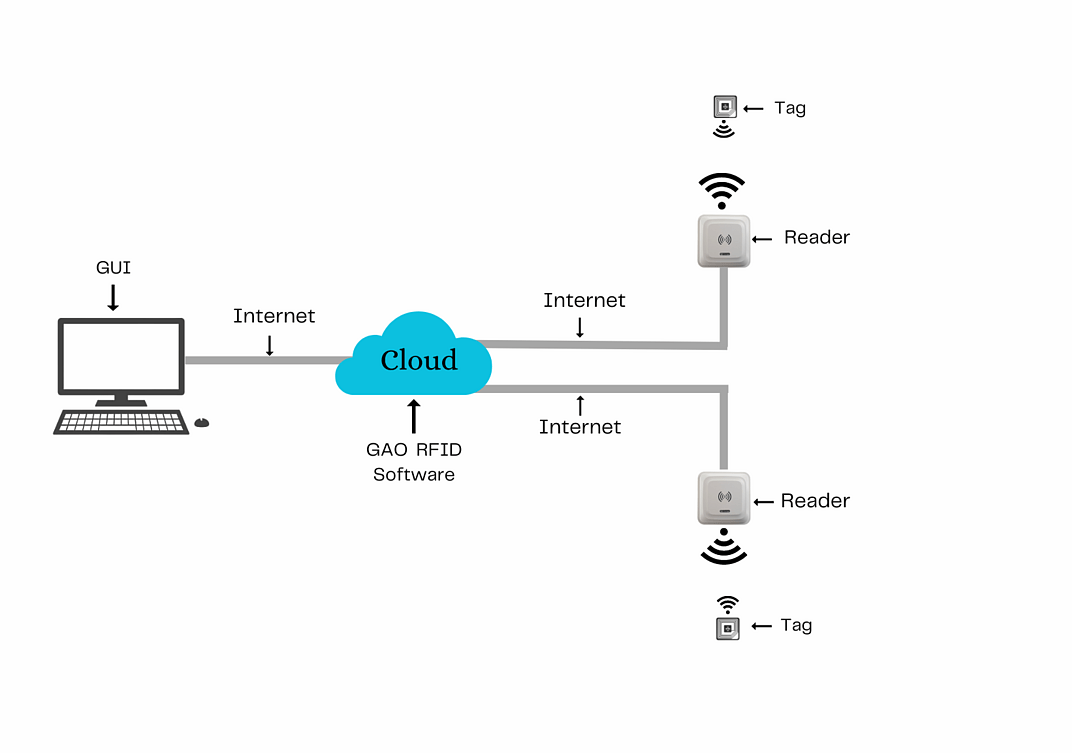
To satisfy its customers, GAO RFID or RFID Systems for pharmaceutical and medicine manufacturing industry are offered in 2 versions. One version is that your software runs on a local server and another version is that your software runs in the cloud. The above illustrates the GAO system for the pharmaceutical and medicine manufacturing with its software running on a local server.
GAO’s RFID technologies bring many benefits to the pharmaceutical and drug manufacturing industry:
- Increased Inventory Management Efficiency: With GAO’s RFID, you’ll be able to automatically identify and track your pharmaceuticals throughout the supply chain. This will improve the visibility of your inventory and reduce the time you spend on manual tracking tasks. We guarantee that you will save time and money!
- Improve the accuracy and speed of product tracking: Our RFID enables automated, real-time tracking of your products, reducing errors and improving the speed of tracking processes. You can count on accurate and updated data at all times to make informed decisions in your business.
- Increase the security and authenticity of the product: By implementing our RFID you will be able to carry out safe and precise tracking of your products, reducing the risk of counterfeiting and improving patient safety. Currently, the safety of pharmaceutical products is a priority in the industry, and we help you guarantee it!
- Improve product traceability: GAO’s RFID allows you to track your products from manufacturing to delivery. This improves the traceability of your products and makes it easier to identify and solve problems in case of quality or safety issues. With our RFID, you will have everything under control!
- Reduced Human Error: With GAO RFID, the need for repetitive manual tasks is eliminated, reducing the likelihood of human error. GAO’s RFID technology helps ensure that each product is in the right place at the right time, preventing errors that could affect the quality and safety of pharmaceutical products.
- Improved efficiency and productivity: By automating many of the inventory tracking and control processes, our RFID can improve production line efficiency and productivity. This allows companies to make more products in less time and with fewer resources, which translates into increased profitability in the long run.
GAO’s BLE technologies offer a longer read range and are particularly attractive for applications with larger workspaces within the pharmaceutical and drug manufacturing industry:
- Real-time monitoring: With GAO’s BLE, you will be able to monitor processes and assets in pharmaceutical and medicine manufacturing in real time. This will allow you to make informed decisions and improve the efficiency of your business.
- Enhanced Security: Our BLE can also be used to enhance security in the workplace. With our technology, you will be able to monitor the location of your workers in real time and take measures to protect them in an emergency. In addition, our GAO BLE can also be used to control access to restricted areas.
- Cost savings: With our BLE, you will be able to reduce maintenance costs and improve energy efficiency in the pharmaceutical and medicine manufacturing. You will also be able to reduce errors and improve efficiency by automating processes and monitoring them remotely.
- Increased Productivity: Our BLE enables low-power wireless communication, improving energy efficiency and extending battery life. This will allow you to increase productivity and reduce maintenance costs in pharmaceutical and medicine manufacturing.
- Resource optimization: With our BLE, you will be able to optimize the use of resources such as materials, equipment and labor in pharmaceutical and medicine manufacturing. Our technology will allow you to monitor the use of these resources and take steps to reduce waste and improve efficiency.
- Data Analysis: GAO’s BLE will also allow you to collect and analyze large amounts of pharmaceutical and medicine manufacturing data. This will give you a clearer vision of how your processes work and you will be able to identify areas for improvement and innovation opportunities in your business.
GAO’s drone and RFID technologies are often combined, and such solutions offer the following benefits to the pharmaceutical and drug manufacturing industry:
- Greater efficiency: With our RFID combining with drones, you will be able to scan and monitor large areas of pharmaceutical and medicine manufacturing in less time than with conventional methods. This will allow you to save time and money and increase the efficiency of your business.
- Higher accuracy: Drone with GAO’s RFID also allows for more accurate data collection. By using RFID-equipped drones, you will be able to gain greater accuracy in locating and tracking pharmaceutical and medicine manufacturing assets.
- Security improvement: With our RFID with drones, you will be able to monitor the security of pharmaceutical and medicine manufacturing in real time. This will allow you to detect and prevent potential security risks and improve safety in the workplace.
- Error reduction: Our RFID with drones can be used to automate processes and reduce human errors. By automating data collection and asset tracking, you can reduce errors and improve pharmaceutical and medicine manufacturing efficiencies.
- Cost savings: GAO’s RFID with drones, you will be able to reduce maintenance costs and improve energy efficiency in pharmaceutical and medicine manufacturing. You will also be able to reduce errors and improve efficiency by automating processes and monitoring them remotely.
- Improved inventory control: Our RFID with drones, you will be able to control inventory more accurately and efficiently. You will be able to know in real time the location of the products, their quantity and their status, which will allow you to make faster and more efficient decisions about the management of your inventory. Plus, you’ll be able to prevent asset loss and reduce replacement costs.
Here are the benefits of GAO’s IoT technologies for the pharmaceutical and drug manufacturing industry:
- Real-time monitoring: With GAO IoT, you will be able to detect and correct problems in real time, which will optimize your operations and reduce downtime, thus increasing the efficiency and profitability of your company.
- Process automation: Thanks to GAO IoT, you will be able to automate repetitive processes and reduce time and errors in production. In addition, automation will allow you to increase the speed of your operations and improve the quality of your products, which will give you a competitive advantage in the market.
- Cost savings: With GAO IoT, you will be able to identify areas where you can reduce the use of energy, materials, and labor, which will reduce production costs and increase your company’s profit margin. With GAO IoT, you will also be able to make data-driven decisions to optimize your operations and reduce costs.
- Product Quality Improvement: GAO IoT will allow you to monitor and control the quality of your products in real time, allowing you to detect quality issues before they become big problems and take action to improve the quality of your products. In this way, you will be able to gain the trust of your customers and improve your reputation in the market.
- Advanced Data Analytics: With GAO IoT, you will be able to collect and analyze large amounts of data in real time to identify patterns and trends in your operations. By making decisions based on data, you will be able to improve the efficiency and profitability of your company and predict and prevent problems before they occur.
- Product Personalization: Thanks to GAO IoT, you will be able to collect data about the preferences and needs of your customers and customize your products to meet their unique needs. This will improve the satisfaction and loyalty of your customers towards your brand and give you a competitive advantage in the market.
GAO’s assist clients with pharmaceutical and drug manufacturing standards, mandates, and regulations
GAO RFID Inc. has developed its products and systems in accordance with the standards and mandates of pharmaceutical and drug manufacturing. GAO has helped our pharmaceutical and drug manufacturing clients implement RFID, BLE, IoT, and drone systems and ensure that such implementations comply with applicable industry standards, US government regulations, and regulations. from the Government of Canada, such as:
RFID, BLE, IoT, & Drone Standards & Mandates
- Ecolabel: Sets global standards for RFID object identification and provides a framework for adoption of the technology in North America.
- FCC: The US Federal Communications Commission regulates the use of radio frequencies used by BLE devices and drones.
- Health Canada: Regulates medical devices in Canada, including IoT and RFID products used in healthcare.
- Industry Canada: Regulates communication and radio communication devices in Canada, including BLE devices and drones.
- FDA: The US Food and Drug Administration regulates the manufacturing, distribution, and sale of pharmaceutical and medical products that use RFID and IoT.
- Transport Canada: Regulates the operation of drones in Canada, including RFID-equipped drones and other IoT devices.
- NIST: The US National Institute of Standards and Technology develops security standards for IoT and RFID devices in the industry.
- ANSI: The American National Standards Institute regulates the adoption of technology standards in the US, including RFID, BLE, and IoT.
- ISO: The International Organization for Standardization sets global standards for emerging technologies, such as IoT and RFID, in industry.
- IEC: The International Electrotechnical Commission sets global standards for emerging technologies, such as IoT and RFID, in industry.
- CSA Group: Develops safety and functional standards for electronic and electrical devices in Canada, including BLE, RFID, IoT, and drones.
- UL: Under its certification program, UL evaluates and certifies the safety and quality of electrical and electronic products, including IoT and RFID devices.
- ICH: is a global organization that develops and promotes harmonized guidelines for the pharmaceutical industry. Their guidelines cover various aspects, including quality management systems, clinical trials, safety, and efficacy testing.
- EMA: is the regulatory agency responsible for the evaluation and supervision of medicines in the European Union. They set standards for drug manufacturing, clinical trials, and quality control.
- USP: is addressing quality assurance, enhancing regulatory predictability, and helping manufacturers distribute quality medicines, dietary supplements and foods
- WHO: The WHO sets international standards and guidelines for pharmaceuticals, including Good Manufacturing Practices (GMP), to ensure the quality, safety, and efficacy of medicines worldwide.
US. Government Regulations
- OSHA: The Occupational Safety and Health Administration is responsible for setting and enforcing workplace safety standards and protecting workers in the US.
- EPA: The Environmental Protection Agency regulates and enforces laws related to the environment and pollution in the US.
- FDA: The Food and Drug Administration regulates and oversees the safety and efficacy of food, drugs, medical devices, and cosmetics in the United States.
- NIOSH: The National Institute for Occupational Safety and Health conducts research and provides recommendations to improve safety and health in the workplace.
- DOT: The US Department of Transportation regulates the transportation of dangerous goods and other products related to transportation safety.
Canadian Government Regulations
- CSA: The Canadian Standards Association sets safety standards and regulations for products and equipment in Canada.
- Health Canada: The government department is responsible for overseeing the safety and effectiveness of medical products, food, and drugs in Canada.
- Environment and Climate Change Canada: The ministry is responsible for applying and enforcing environmental laws and air and water quality standards in Canada.
- Transport Canada: The department is responsible for regulating and overseeing transportation safety in Canada.
- Natural Resources Canada: The ministry promotes the development and sustainable use of Canada’s natural resources, including energy and minerals
GAO software provides easy API integration
GAO’s RFID and BLE software offers a free trial for both server-based and cloud-based versions, and offers an API for important systems in the production of pharmaceuticals and medicines, such as:
Personnel management:
- Payroll and benefits administration
- Management of schedules and shifts
- Job security and training
Team management:
- Scheduling and preventive maintenance
- Machinery and equipment safety
- Performance monitoring and analysis
Access control:
- Identification and authentication of employees
- Access control to sensitive areas and clean rooms
- Time and attendance record
Warehouse management:
- Inventory control and lot tracking
- Order management and order fulfillment
- Storage space optimization
Supply chain management:
- Supply Chain Track and Trace
- Quality control and quality assurance
- Demand planning and supply chain management
Other applications:
- Environmental monitoring to ensure product safety and quality
- Data analysis and data-driven decision making to optimize production and efficiency
- Regulatory compliance and monitoring of the regulation of the FDA and other regulatory entities.
GAO has integrated its RFID, BLE, IoT and drone systems with some of the leading software and cloud services in the pharmaceutical and drug manufacturing industry. Below are some of the popular software and cloud services in the pharmaceutical and drug manufacturing industry.
- Oracle SCM Cloud: An end-to-end supply chain management cloud solution with track and trace capabilities for pharmaceuticals.
- SAP EWM: A warehouse management system that helps optimize the efficiency of inventory management and quality control in the pharmaceutical industry.
- Infor CloudSuite WMS: A cloud-based warehouse management software that improves the efficiency and accuracy of pharmaceutical inventory management.
- Workday HCM: A workforce management solution that includes talent management, payroll, and time and attendance tracking for the pharmaceutical industry.
- Zenefits: An online HR platform that simplifies benefits, time off and regulatory compliance management for the pharmaceutical industry.
- Salesforce Health Cloud: A customer relationship management platform that focuses on the healthcare industry, providing patient and pharmaceutical tracking and analytics.
- Microsoft Dynamics 365 Supply Chain Management: A supply chain management solution that includes features for production planning and control in the pharmaceutical industry.
- JDA Software: A supply chain planning solution that provides real-time visibility and improves the efficiency of inventory management in the pharmaceutical industry.
- Deloitte Digital: An online consulting solution that helps companies in the pharmaceutical industry optimize supply chain efficiencies and improve the customer experience.
- IQMS Manufacturing ERP: an enterprise resource planning software that helps manage production, inventories, and costs in the pharmaceutical industry.
- Cognizant Life Sciences: A digital services platform that helps supply chain management and implementation of manufacturing solutions for the pharmaceutical industry.
- TraceLink: A track and trace solution that uses blockchain technology to ensure the integrity and security of the pharmaceutical supply chain.
GAO has worked with some of the leading pharmaceutical and drug technology companies to provide integrated RFID, BLE, IoT and drone solutions to clients. These are some of the technological leaders in the industry of the production of pharmaceutical products and medicines:
- Siemens Healthineers: Provides medical diagnosis and treatment solutions, including medical devices and imaging systems.
- Microsoft: Provides cloud services, artificial intelligence solutions, and business software for the pharmaceutical industry.
- Pfizer: Leading biopharmaceutical company that develops, produces, and distributes medicines and vaccines.
- Emerson: Manufactures equipment and automation systems to produce pharmaceuticals and medicines.
- Roche: Biopharmaceutical company that focuses on the diagnosis and treatment of diseases and medical disorders.
- Honeywell: Provides industrial automation solutions and software for the pharmaceutical and drug manufacturing industry.
- Oracle: Provides enterprise software, cloud services, and artificial intelligence solutions for the pharmaceutical industry.
- Merck: Biopharmaceutical company that focuses on the development, production and distribution of medicines and vaccines.
- IBM: Offers artificial intelligence solutions, cloud services and business software for the pharmaceutical industry.
- 3M: Manufactures and supplies a wide range of products for the pharmaceutical and drug manufacturing industry, including personal protective equipment and automation solutions.
- Thermo Fisher Scientific: Offers a wide range of products and services for the research, diagnosis and production of pharmaceuticals and medicines.
- Johnson & Johnson: A leading biopharmaceutical company that develops, produces and distributes a broad range of medical and pharmaceutical products, including drugs and medical devices.
Case Studies of RFID Applications
Below are some RFID application cases in the industry:
Pfizer implemented an RFID-based track-and-trace system for its pharmaceutical products to improve the visibility and traceability of its supply chain. The system uses UHF RFID tags and readers to track products as they move through the supply chain, from manufacturing to distribution to the end customer. This allows Pfizer to quickly identify and address any issues that may arise, such as product recalls or counterfeiting.
GlaxoSmithKline implemented an RFID-enabled system to track and monitor the temperature of its vaccines during distribution. The system uses UHF RFID tags and readers to track the temperature of the vaccines as they are shipped from the manufacturing site to the distributor and then to the healthcare facility. This ensures that the vaccines are kept at the appropriate temperature throughout the supply chain, which is critical to their efficacy.
Apotex, a Canadian pharmaceutical company, implemented an RFID-enabled inventory management system to improve the efficiency of its warehouse operations. The system uses UHF RFID tags and readers to track inventory as it moves in and out of the warehouse. This allows Apotex to quickly locate and retrieve products, reducing the time and labor required for inventory management.
Eli Lilly implemented an RFID-enabled system to track the movement of its pharmaceutical products through the manufacturing process. The system uses UHF RFID tags and readers to track products as they move from one production area to another. This allows Eli Lilly to quickly identify and address any bottlenecks or delays in the manufacturing process, improving efficiency and reducing production costs.
GAO RFID Systems & Hardware for the manufacture of pharmaceuticals and medicines
GAO RFID Inc. offers the largest selection of BLE gateways, BLE beacons, RFID readers, tags, antenna, printers, and integrated RFID systems for various industries, including the pharmaceutical and medicine manufacturing.
BLE (Bluetooth Low Energy)
GAO offers advanced BLE gateways:
as well as versatile beacons with such important functions as temperature, humility, vibration and panic button:
GAO’s BLE technology is suitable for many industries, including the Pharmaceutical and medicine manufacturing.
UHF (Ultra High Frequency) RFID
GAO offers the largest selection of UHF RFID readers for various industries, including the Pharmaceutical and medicine manufacturing:
GAO RFID offers the widest choice of UHF RFID tags, labels, badges, wristbands for various industries, including the Pharmaceutical and medicine manufacturing:
and an array of antennas to address different applications:
HF (High Frequency), NFC (Near Field Communications) and LF (Low Frequency) RFID
GAO offers the largest selection of HF, NFC, and LF RFID readers for various industries, including the Pharmaceutical and medicine manufacturing:
- High Frequency 13.56 MHz Passive RFID Readers
- Low Frequency 134 kHz Passive RFID Readers
- Low Frequency 125 kHz Passive RFID Readers
HF, NFC and LF RFID tags, labels, badges, wristbands for various industries, including the Pharmaceutical and medicine manufacturing:
and antennas:
GAO also offers RFID printers:
Digital I/O adapters:
and relay controllers:
For embedded applications, GAO offers UHF, HF and LF RFID reader modules:
- UHF 860 – 960 MHz RFID Modules
- 13.56 MHz High Frequency RFID Modules
- 125 kHz Low Frequency RFID Modules
The RFID systems by GAO are highly popular for clients in the Pharmaceutical and medicine
People or workers tracking system:
Assets that can be effectively tracked using GAO’s technologies include
- Autoclaves: Used for sterilizing equipment, containers, and media through high-pressure steam.
- Incubators: Used for maintaining controlled temperature and humidity conditions to cultivate microorganisms or conduct stability testing.
- Fermenters and Bioreactors: Used for large-scale production of biological substances such as vaccines, antibiotics, and enzymes using microbial or cell cultures.
- Lyophilizers: Also known as freeze dryers, they remove moisture from products while preserving their structure and biological activity.
- Milling Machines: Used for reducing the particle size of pharmaceutical ingredients and improving solubility, dissolution, and bioavailability.
- Mixers and Blenders: Used to homogeneously blend different ingredients, such as powders, granules, or liquids, in the formulation of drugs.
- Tablet Press Machines: Used to compress granulated powders into tablets with precise weights and shapes.
- Capsule Filling Machines: Used for filling empty gelatin or vegetarian capsules with powdered or granulated pharmaceutical ingredients.
- Coating Machines: Used to apply protective or functional coatings to tablets, capsules, or granules to control drug release or enhance appearance.
- Dissolution Testers: Used to measure the rate at which a drug substance dissolves from a solid dosage form, ensuring consistent performance.
- High-Performance Liquid Chromatography (HPLC) Systems: Analytical instruments used to separate, identify, and quantify pharmaceutical compounds in a sample.
- Gas Chromatography (GC) Systems: Used for analyzing volatile compounds, including residual solvents or impurities in drug formulations.
- Mass Spectrometers: Instruments used to determine the molecular composition and structure of pharmaceutical compounds.
- Particle Size Analyzers: Devices used to measure the size distribution of particles in pharmaceutical formulations.
- Spectrophotometers: Used for analyzing the concentration of a substance in a solution, measuring absorbance or transmittance of light.
- Sterile Isolators: Enclosed systems used to handle aseptic operations and protect the product from contamination.
- HVAC Systems: Specialized heating, ventilation, and air conditioning systems to maintain controlled environments and prevent cross-contamination.
Physical asset or operational equipment tracking system:
Personnel or people access control system:
Parking or vehicle control system:
GAO has served the pharmaceutical and medicine manufacturing extensively
GAO’s products and technologies have helped clients in the pharmaceutical and drug manufacturing industry achieve success in Artificial Intelligence, Big Data, Industry 4.0, Blockchain, Additive Manufacturing, Personalization, Sustainability, Pharmacovigilance.
GAO RFID Inc. has served many clients in the production of pharmaceuticals and medicines, including its various divisions such as
- Research and Development (R&D): This division focuses on the discovery and development of new drugs, therapies, and medical treatments. It involves extensive research, preclinical studies, clinical trials, and regulatory approvals.
- Drug Formulation and Development: This division is responsible for formulating drugs into various dosage forms, such as tablets, capsules, injections, creams, and liquids. It involves developing the appropriate drug delivery systems and ensuring stability, bioavailability, and patient compliance.
- Active Pharmaceutical Ingredient (API) Manufacturing: API manufacturing involves the production of the active substances or compounds that have therapeutic effects. APIs are the key ingredients in pharmaceutical products and are typically produced through chemical synthesis or biotechnological processes.
- Generic Pharmaceuticals: This division focuses on the manufacturing of generic drugs, which are bioequivalent versions of brand-name drugs that have gone off-patent. Generic pharmaceutical companies replicate the established formulas and manufacturing processes of the original drugs.
- Biopharmaceuticals: Biopharmaceuticals, also known as biologics, are drugs produced using biotechnology processes. This division involves the manufacturing of complex molecules derived from living organisms, such as proteins, antibodies, vaccines, and gene therapies.
- Over-the-Counter (OTC) Medications: OTC medications are non-prescription drugs that are widely available for self-medication. This division involves the manufacturing of OTC products, including pain relievers, cough and cold medicines, digestive aids, and skincare products.
- Contract Manufacturing and Outsourcing: Many pharmaceutical companies outsource certain manufacturing processes to specialized contract manufacturing organizations (CMOs). This division focuses on providing manufacturing services, including formulation, packaging, and quality control, on behalf of pharmaceutical companies.
- Medical Device Manufacturing: While not strictly part of the pharmaceutical industry, medical device manufacturing is closely associated with pharmaceutical companies. This division involves the production of medical devices, equipment, and diagnostic tools used in healthcare, such as surgical instruments, implants, imaging systems, and diagnostic kits.
- Regulatory Affairs: Regulatory affairs departments ensure compliance with the relevant laws, regulations, and quality standards in the pharmaceutical industry. They are responsible for obtaining regulatory approvals, managing documentation, and ensuring product safety and efficacy.
- Quality Control and Quality Assurance: This division focuses on maintaining the quality and safety of pharmaceutical products. It involves quality testing, monitoring manufacturing processes, ensuring compliance with quality standards, and implementing quality management systems.
Here are some of the leading companies in the pharmaceutical and medical manufacturing