
Overview
The surgical and medical instrument manufacturing industry is a sector of the healthcare and medical device manufacturing field that specializes in the production of a wide range of instruments, tools, and equipment used in medical and surgical procedures. This industry plays a crucial role in providing healthcare professionals with the necessary tools to diagnose, treat, and care for patients effectively. The surgical and medical instrument manufacturing industryin GAO’s is a vital component of the healthcare ecosystem, responsible for producing a wide range of essential medical tools and devices used in diagnosis, treatment, and patient care. It operates in a competitive and highly regulated environment, with a constant focus on quality, innovation, and global market dynamics.
GAO’s RFID, BLE, IoT, and drone technologies have helped its customers in the surgical and medical instrument manufacturing industry to improve their work processes, their operations and productivity by better management of their staff, materials and operational equipment such as CNC machines for precise machining, laser cutting machines for precision cutting, and engraving, injection molding machines for plastic components, 3D printing and additive manufacturing equipment for prototypes and custom implants, cleanrooms and sterilization equipment for maintaining cleanliness and ensuring product sterility, quality control and testing equipment for assurance, and various tools for assembly, packaging, and surface treatment. Additionally, CNC Swiss screw machines, grinding machines, microscopy systems, metrology equipment, CAD/CAM software, and material handling systems are vital components of the manufacturing process. Environmental control systems, labeling and printing machines, and water purification systems also play essential roles in this industry.
Ranked as one of the top 10 global RFID suppliers, GAO RFID Inc. is based in New York City, U.S. and Toronto, Canada. GAO offers a comprehensive selection of UHF, HF (including NFC) and LF RFID (radio frequency identification) readers and tags, BLE (Low Energy Bluetooth) gateways and beacons, and various RFID and BLE systems such as people tracking, asset tracking, access control, parking control, fleet management, WIP (work in progress), traceability. Such RFID and BLE products and systems, as well as its IoT and drone technologies, have been successfully deployed for surgical and medical instrument manufacturing industry. Its sister company, GAO Tek Inc. https://gaotek.com, is a leading supplier of industrial or commercial testers and analyzers, drones, and network products.
The targeted markets of both GAO RFID Inc. and GAO Tek Inc. are North America, particularly the U.S., Canada, Mexico, and Europe. As a result, this website gaorfid.com is offered in English and other major languages of North America and Europe such as Spanish, French, German, Italian, Polish, Ukrainian, Romanian, Russian, Dutch, Turkish, Greek, Hungarian, Swedish, Czech, Portuguese, Serbian, Bulgarian, Croatian, Danish, Finnish, Norwegian, Slovak, Catalan, Lithuanian, Bosnian, Galician, Slovene, Latvian, Estonian, Welsh, Icelandic, and Irish.
Applications & Benefits of GAO’s RFID, BLE, IoT & Drones for Surgical and Medical Instrument Manufacturing
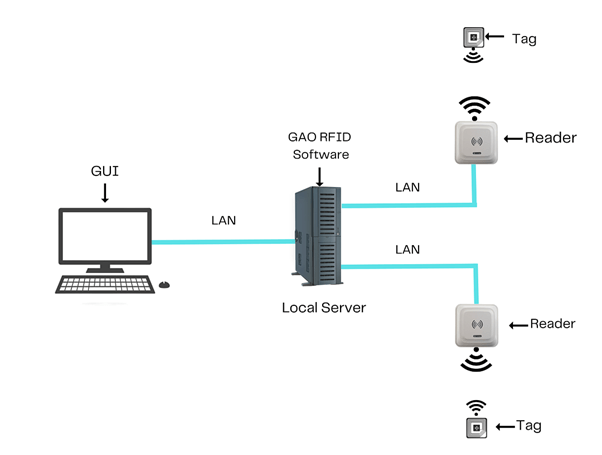 To satisfy its customers, GAO’s RFID or RFID Systems for surgical and medical instrument manufactures are offered in 2 versions. One version is that its software is running on a local server that normally is on our client’s premises, and another version runs in the cloud. The cloud server could be GAO’s cloud server, client’s own cloud server or a cloud server from one of the leading cloud server providers such as Amazon Web Services (AWS), Microsoft Azure, Google Cloud, IBM Cloud (formerly SoftLayer), Oracle Cloud, RedHat, Heroku, Digital Ocean, CloudFlare, Linode and Rackspace. The above illustrates GAO system for surgical and medical instrument manufacturing software running on a local server.
To satisfy its customers, GAO’s RFID or RFID Systems for surgical and medical instrument manufactures are offered in 2 versions. One version is that its software is running on a local server that normally is on our client’s premises, and another version runs in the cloud. The cloud server could be GAO’s cloud server, client’s own cloud server or a cloud server from one of the leading cloud server providers such as Amazon Web Services (AWS), Microsoft Azure, Google Cloud, IBM Cloud (formerly SoftLayer), Oracle Cloud, RedHat, Heroku, Digital Ocean, CloudFlare, Linode and Rackspace. The above illustrates GAO system for surgical and medical instrument manufacturing software running on a local server.
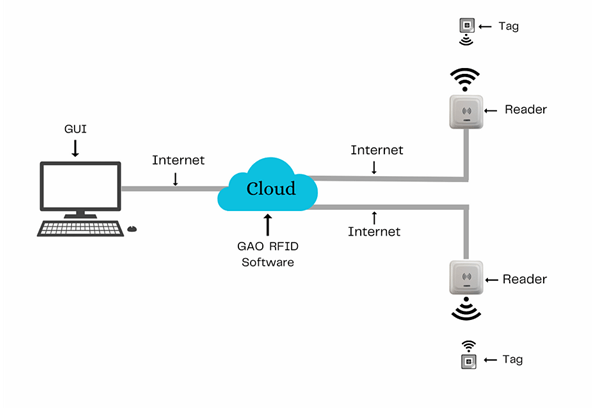 The above illustrates GAO system for surgical and medical instrument manufacturing with its software running in cloud.
The above illustrates GAO system for surgical and medical instrument manufacturing with its software running in cloud.
GAO’s RFID and BLE technologies, consisting of RFID readers, RFID tags, BLE gateways, BLE beacons, software, cloud services and their systems, have the following applications in surgical and medical instrument manufacturing industry:
- Asset Tracking:GAO’s RFID tags can be attached to surgical instruments, equipment, and medical devices to track their location and usage within a healthcare facility. This helps in inventory management, reduces loss or misplacement, and ensures that instruments are readily available when needed.
- Inventory Management: GAO’s RFID enables real-time monitoring of inventory levels for medical supplies, instruments, and devices. This streamlines the supply chain, reduces overstocking or stockouts, and helps in optimizing inventory levels to minimize costs.
- Sterilization Tracking: GAO’s RFID tags can be used to monitor the sterilization process of medical instruments. Each instrument can have an RFID tag that records when it was sterilized, ensuring compliance with safety standards and regulations.
- Patient Safety: GAO’s RFID technology can be employed to verify that the correct instruments and devices are used for specific patients during surgeries and medical procedures, reducing the risk of errors.
- Quality Control: GAO’s RFID tags can store information about the manufacturing process and materials used in medical instruments. This data can be accessed to verify the quality and authenticity of products, helping in quality control and traceability.
- Maintenance and Calibration: GAO’s RFID can aid in tracking the maintenance and calibration schedules of medical equipment and instruments, ensuring they remain in proper working condition and meet regulatory requirements.
GAO’s drone technologies find the following applications in the surgical and medical instrument manufacturing industry:
- Inventory Management: GAO’s Drones can be used for aerial inventory tracking and management in manufacturing facilities, helping to monitor the stock levels of raw materials and components required for medical instrument production.
- Facility Inspections: GAO’s Drones equipped with cameras and sensors can conduct routine inspections of manufacturing facilities, identifying maintenance needs, safety hazards, and potential issues that may impact production processes.
- Quality Control: GAO’s Drones equipped with high-resolution cameras can perform quality control checks by capturing detailed images of medical instruments and devices, allowing for rapid and thorough visual inspections.
- Environmental Monitoring: GAO’s Drones can assess environmental conditions within manufacturing facilities, including temperature, humidity, and air quality, ensuring that production processes meet the required standards for medical device manufacturing.
- Security and Surveillance: GAO’s Drones can enhance security by monitoring manufacturing facilities for unauthorized access, theft, or vandalism, helping to protect valuable equipment and products.
- Transport and Delivery: GAO’s Drones can be used for the rapid and secure transport of small, high-value medical instruments and components between manufacturing facilities or to healthcare facilities.
GAO’s IoT technologies, consisting of IoT sensors, sensors networks and systems, find the following applications in the surgical and medical instrument manufacturing industry:
- Remote Monitoring of Equipment: GAO’s IoT sensors can be integrated into manufacturing equipment and machinery to monitor their status in real-time. This enables predictive maintenance, reducing downtime and ensuring that manufacturing processes run smoothly.
- Quality Control: GAO’s IoT sensors can track, and record data related to the manufacturing process, helping to maintain consistent product quality. For example, sensors can monitor temperature, pressure, and other variables to ensure that instruments are fabricated to precise specifications.
- Asset Tracking: GAO’s RFID tags or GPS-enabled IoT devices can be used to track the location and status of equipment, materials, and finished products throughout the manufacturing and supply chain process, enhancing visibility and inventory management.
- Supply Chain Management: GAO’s IoT technologies can optimize the supply chain by providing real-time data on the movement of raw materials, components, and finished products. This can help in reducing lead times, minimizing waste, and improving inventory control.
- Environmental Monitoring: GAO’s IoT sensors can monitor environmental conditions within manufacturing facilities, ensuring compliance with regulatory standards for temperature, humidity, and air quality, which are critical for medical device production.
- Worker Safety: GAO’s wearable IoT devices can monitor the health and safety of workers, ensuring they are not exposed to hazardous conditions and are following safety protocols. This is particularly important in environments where exposure to chemicals or radiation is a concern.
- Process Automation: GAO’s IoT can be used to automate various aspects of the manufacturing process, including material handling, assembly, and quality control, leading to increased efficiency and reduced human error.
GAO Helps Customers Comply with Standards, Mandates & Regulations of Surgical and Medical Instrument Manufacturing industry
GAO RFID Inc. has helped many companies in surgical and medical instrument manufacturing industry to deploy RFID, BLE, IoT and drone systems and to ensure such deployments complying with the applicable industry standards, mandates and government regulations:
RFID, BLE, IoT, & Drone standard & mandates
- ISO 15693
- ISO 18000-3
- ISO 28560
- ASTM F38
- ISO 21384-3
- HL7 (Health Level Seven)
- AAMI TIR69
- ASTM F2273
- IEC 60601-1-6
- GS1 EPCIS (Electronic Product Code Information Services)
- FDA UDI (Unique Device Identification)
- Aviation Authority Regulations
- Remote Pilot Certification
- Data Privacy and Security
- Cybersecurity Guidelines
- Interoperability Standards
- Medical Device Regulations
- Wireless Communication Regulations
U.S. Government Regulations
- FDA Quality System Regulation (QSR) – 21 CFR Part 820
- FDA Medical Device Reporting (MDR) – 21 CFR Part 803
- FDA Premarket Notification (510(k)) – 21 CFR Part 807
- FDA Unique Device Identification (UDI) – 21 CFR Part 830
- FDA Investigational Device Exemption (IDE) – 21 CFR Part 812
- CDC Guidelines for Sterilization and Disinfection
- DEA Controlled Substances Regulations – 21 CFR Parts 1300-1316
- NIST Cybersecurity Framework
- EPA Hazardous Waste Regulations – 40 CFR Parts 260-279
- BIS Export Controls (for international sales)
Canadian Government Regulations
- Medical Devices Regulations (MDR) – SOR/98-282
- Health Canada Medical Devices Guidance Documents
- Health Canada Medical Device Licensing for Investigational Testing
- Canada Border Services Agency (CBSA) Import and Export Regulations
- Health Canada Regulations for Combination Products
- Transport Canada Regulations for Shipping Hazardous Materials
- Pest Control Products Act (PCPA) Regulations for Sterilization and Disinfection
GAO Software Provides Easy Integration with API
GAO’s RFID and BLE software offers a free trial for both the server-based and cloud versions, and offers an API to the important systems in surgical and medical instrument manufacturing such as:
Personnel Management:
- Employee Records and HR Management
- Workforce Scheduling and Shift Management
- Training and Certification Tracking
- Health and Safety Compliance Monitoring
- Payroll and Compensation Management
Equipment Management:
- Maintenance Scheduling and Predictive Maintenance
- Equipment Calibration and Testing
- Equipment Utilization Analytics
- Equipment Fault Detection and Alerts
Access Control:
- Visitor Management and Access Logs
- Document and Data Access Control
- Biometric Authentication for Secure Access
Warehouse Management:
- Order Management and Fulfillment
- Quality Control and Inspection
- Real-time Location Systems (RTLS) for Asset Tracking
- Vendor Managed Inventory (VMI)
Supply Chain Management:
- Demand Forecasting and Inventory Planning
- Cold Chain Monitoring (for temperature-sensitive products)
- Transportation Management and Route Optimization
- Regulatory Compliance Tracking
Other Applications:
- Statistical Process Control (SPC)
- Non-destructive Testing (NDT)
- Real-time Monitoring Dashboards
- CAPA (Corrective and Preventive Actions) Tracking
- Electronic Batch Records (EBR) Compliance Documentation Storage
GAO has enabled its customers to make use of some of the leading software and cloud services in the surgical and medical instrument manufacturing industry. Below are some of the popular software and cloud services in surgical and medical instrument manufacturing.
Workday, SAP SuccessFactors, Oracle HCM Cloud, Ultimate Software UltiPro, and ADP Workforce Now. For equipment management, notable options UpKeep, Infor EAM, MPulse, Fiix. Access control solutions encompass HID Global, LenelS2, Honeywell Access Control and Kisi. In the realm of warehouse management, top choices consist of SAP Extended Warehouse Management (EWM), HighJump Warehouse Management System (WMS), Oracle Warehouse Management Cloud and Infor CloudSuite WMS. In supply chain management, JDA Software (now Blue Yonder), Llamasoft Supply Chain Guru, Kinaxis RapidResponse.
GAO has worked with some of the leading technology companies surgical and medical instrument manufacturing industries to provide integrated RFID, BLE, IoT and drone solutions to customers. Here are some of the technology leaders in surgical and medical instrument manufacturing industry.
IBM, Oracle, Microsoft, Philips Healthcare, Siemens Healthineers, Cerner Corporation, Epic Systems Corporation and BD (Becton, Dickinson, and Company).
Case Studies of RFID, IoT & Drone Applications
Case Studies of RFID Applications:
Below are some RFID application cases in surgical and medical instrument manufacturing industry.
A case study on how RFID technology is being used to track and manage sterile surgical instruments throughout the manufacturing process. This could include tracking instruments from production to packaging and ensuring that they remain sterile until they are used in surgery.
A case study on how RFID technology assists medical instrument manufacturers in meeting regulatory requirements by providing accurate traceability and documentation throughout the production process.
A case study shows how UHF RFID is used in the USA to enhance inventory management processes for medical instrument manufacturers, including tracking raw materials, work-in-progress (WIP), and finished products in real-time.
In Canada, RFID implementations in medical instrument manufacturing sector that focus on improving inventory management processes, including tracking raw materials, work-in-progress (WIP), and finished goods.
A case study shows how RFID is used in Mexican healthcare facilities to track and manage sterile surgical instruments, ensuring their cleanliness and availability for medical procedures.
Many applications of RFID by GAO can be found here.
Case Studies of IoT Applications:
Below are some IoT application cases in the surgical and medical instrument manufacturing industry.
A case study in Canada shows how IoT technology is used in Canada to create connected medical devices that collect and transmit patient data to healthcare providers for remote monitoring and diagnosis.
Some case study shows the demonstration of the use of IoT sensors in Mexican medical instrument manufacturing facilities to predict equipment failures and optimize maintenance schedules.
A case study shows how IoT applications in Mexican medical instrument manufacturing help maintain and ensure the quality of instruments during production, including real-time data capture and analysis.
Some case studies have demonstrations of IoT solutions in Europe that track the movement and condition of medical instruments through the supply chain, ensuring quality and compliance.
A case study shows how IoT-based security solutions in European healthcare facilities enhance authentication, access control, and data security, particularly for sensitive medical instruments.
Case Studies of Drone Applications:
Below are some drone application cases in the surgical and medical instrument manufacturing industry.
A case study shows a leading medical device manufacturer is using drones to deliver medical supplies to hospitals and other healthcare facilities. The company has partnered with Zipline, a drone delivery company, to operate a drone delivery network in North Carolina. The network delivers a variety of medical supplies, including implants, surgical instruments, and medications.
In USA, a global healthcare company is using drones to deliver vaccines and other medical supplies to remote and underserved communities. The company has partnered with Gavi, the Vaccine Alliance, to operate a drone delivery network in Rwanda. The network has delivered over 1 million doses of vaccines since its launch in 2016.
A medical device manufacturer that specializes in spine surgery is using drones to deliver surgical instruments and other supplies to hospitals and other healthcare facilities. The company has partnered with Zipline to operate a drone delivery network in North Carolina and Arizona. The network delivers a variety of medical supplies, including spine implants, surgical instruments, and medications.
A company in Mexico is working with several Mexican medical device manufacturers to develop drone-based systems for inspecting their products for defects. The company is also working with the Mexican government to develop regulations for the use of drones in the medical device industry.
A case study shows in the Netherlands is using drones to deliver blood products to hospitals in the region. The drone delivery service has been operational since 2017 and has delivered over 10,000 blood products to date.
GAO RFID Systems & Hardware for Surgical and Medical Instrument Manufacturing Industry
GAO RFID Inc. offers the largest selection of BLE gateways, BLE beacons, RFID readers, tags, antenna, printers, and integrated RFID systems for various industries, including surgical and medical instrument manufacturing.
BLE (Bluetooth Low Energy)
GAO offers advanced BLE gateways:
as well as versatile beacons with such important functions as temperature, humility, vibration and panic button:
GAO’s BLE technology is suitable for many industries, including surgical and medical instrument manufacturing.
UHF (Ultra High Frequency) RFID
GAO offers the largest selection of UHF RFID readers for various industries, including surgical and medical instrument manufacturing:
GAO RFID offers the widest choice of UHF RFID tags, labels, badges, wristbands for various industries, including surgical and medical instrument manufacturing:
and an array of antennas to address different applications:
HF (High Frequency), NFC (Near Field Communications) and LF (Low Frequency) RFID
GAO offers the largest selection of HF, NFC, and LF RFID readers for various industries, including surgical and medical instrument manufacturing
HF, NFC and LF RFID tags, labels, badges, wristbands for various industries, including surgical and medical instrument manufacturing:
and antennas:
GAO also offers RFID printers:
Digital I/O adapters:
and relay controllers:
For embedded applications, GAO offers UHF, HF and LF RFID reader modules:
- UHF 860 – 960 MHz RFID Modules
- 13.56 MHz High Frequency RFID Modules
- 125 kHz Low Frequency RFID Modules
In collaboration with its sister company GAO Tek Inc, a wide selection of high-quality drones is offered:
The RFID systems by GAO are highly popular for clients in surgical and medical instrument manufacturing:
Physical asset or operational equipment tracking system:
Assets that can be effectively tracked using GAO’s technologies include tracking solutions, offer versatile asset tracking capabilities across diverse industries. With their RFID technology, GAO effectively tracks a wide array of assets, including inventory items, equipment, tools, medical instruments, vehicles, livestock, documents, retail merchandise, containers, personnel, data center assets, library books, waste bins, high-value artifacts, returnable transit items (RTIs), energy meters, retail assets, hospital equipment pharmaceuticals, and even weapons and military assets. This extensive range of applications demonstrates the flexibility and utility of GAO’s RFID and tracking technologies in optimizing inventory management, enhancing security, and improving operational efficiency across various sectors.
People or workers tracking system:
Personnel or people access control system:
Parking or vehicle control system:
Furthermore, GAO provides the customization of RFID tags, RFID readers, BLE beacons and BLE gateways, IoT, drones, and systems and consulting services for surgical and medical instrument manufacturing and for various industries in all metropolitans in North America, particularly the U.S., Canada and Mexico, and Europe:
GAO makes Efforts To Satisfy Costumer
Large Choice of Products
In order to satisfy the diversified needs of their corporate customers, GAO RFID Inc. and its sister company GAO Tek Inc. together offer a wide choice of RFID, BLE, IoT, drones, testing and measurement devices, and network products.
Overnight Delivery
In order to shorten the delivery to our customers, GAO has maintained a large stock of its products and is able to ship overnight within continental U.S. and Canada, and fast delivery to anywhere in Mexico and Europe from the nearest warehouse.
Local to Our Customers
We are located in both the U.S. and Canada. We travel to customers’ premises if necessary. Hence, we provide a very strong local support to our customers in North America, particularly the U.S., Canada and Mexico, and Europe. Furthermore, we have built partnerships with some integrators, consulting firms and other service providers in different cities to further strengthen our services. Here are some of the service providers in surgical and medical instrument manufacturing we have worked with to serve our joint customers:
- ZIBM Global Services
- Deloitte
- Capgemini
- Cognizant
- Accenture
- Wipro
- Tata Consultancy Services (TCS)
- Infosys
- DXC Technology
- HCL Technologies
- Tech Mahindra
- Cerner
- Softtek
- Neoris
- KIO NetworksPwC CanadaCGIAtos
GAO Has Served Surgical and Medical Instrument Manufacturing Extensively
GAO RFID Inc. and its sister company GAO Tek Inc. together offer a wide choice of RFID, BLE, IoT, drone, testing and measurement devices, and network products.
GAO’s products and technologies have helped its customers in surgical and medical instrument manufacturing industry to achieve success in
Industry 4.0, additive manufacturing (3D printing), the Internet of Things (IoT), Personalized medicine and the utilization of artificial intelligence (AI), image recognition, wearable medical devices, telemedicine, miniaturization, Sustainability and supply chain resilience, blockchain and 5G connectivity.
GAO RFID Inc. has deployed RFID, BLE and IoT projects for many companies in surgical and medical instrument manufacturing industry, including many in its various divisions such as:
- Surgical Instruments: Companies in this sub-industry manufacture a wide range of surgical instruments, including scalpels, forceps, scissors, retractors, and clamps used in various medical procedures.
- Dental Instruments: Dental instrument manufacturers produce tools and devices used by dentists and dental professionals for dental procedures, orthodontics, and oral surgery.
- Orthopedic Instruments: This division specializes in the production of instruments used in orthopedic surgery, such as bone saws, drills, and implants for joint replacement procedures.
- Ophthalmic Instruments: Ophthalmic instrument manufacturers produce devices for eye surgery and examinations, including instruments for cataract surgery and ophthalmoscopes.
- Neurosurgical Instruments: Companies in this sub-industry manufacture instruments for neurosurgery, such as cranial drills, microscopes, and specialized tools for brain and spinal cord procedures.
- Cardiovascular Instruments: Manufacturers in this division produce instruments used in cardiovascular surgery, including heart valves, stents, and catheters for diagnostics and interventions.
- Electrosurgical Instruments: Manufacturers in this category produce electrosurgical devices that use electrical energy for cutting, coagulating, and tissue ablation during surgery.
- Diagnostic and Monitoring Equipment: This division includes companies that produce medical devices for diagnosis and monitoring, such as blood pressure monitors, ECG machines, and patient monitoring systems.
- Imaging Equipment: Manufacturers of imaging equipment, including X-ray machines, MRI scanners, and ultrasound devices, play a crucial role in medical diagnostics.
- Rehabilitation and Physical Therapy Equipment: This sub-industry produces equipment like physical therapy machines, mobility aids, and devices used for rehabilitation and physiotherapy.
- Implantable Medical Devices: Manufacturers in this division produce implantable devices like pacemakers, artificial joints, and cochlear implants used to treat various medical conditions.
- Veterinary Instruments: This sub-industry focuses on instruments and equipment used in veterinary medicine, including surgical tools, diagnostic devices, and imaging equipment for animals.
GAO’s technologies enable its customers surgical and medical instrument manufacturing industry to effectively track their workforces such as employee attendance and time tracking , access control and security, inventory management, data security and access monitoring, asset tracking, productivity analysis, quality control and assurance and effectively track operational assets such as supply chain monitoring, asset maintenance, security and authentication, data analytics and logistics and distribution.
Here are some of the leading companies in surgical and medical instrument manufacturing industry GAO has served:
- BD (Becton, Dickinson and Company)
- Stryker Corporation
- Thermo Fisher Scientific
- Zimmer Biomet
- Siemens Healthineers
- Hologic
- Terumo Cardiovascular Group
- Teleflex Incorporated
- CONMED Corporation
- R. Bard, Inc. (A Becton, Dickinson company)
- Johnson & Johnson Medical Devices Companies
- Smith & Nephew
- Arthrex
- Haemonetics Corporation
- Olympus Corporation of the Americas
- CryoLife
- Medtronic
- Abbott Laboratories
- STERIS Corporation
- Cook Medical
- Cretex Companies
- Nordson Medical
- Minnetronix Medical
- DentSply Sirona



















 .
.




You Are Invited to Contact Us!
If you are interested in our products, services or partnering with us, please feel free to contact us by filling out this form:
or email us at sales@gaorfid.com
